Characteristics of slush hydrogen
Since liquid hydrogen’s density and latent heat of vaporization are approximately
1/14 and 1/5 of those of water, respectively, fuel storage tank capacities
become greater for rockets and fuel cells. Also, due to boil-off resulting
from heat inleak during transport and storage, the reduction in transport
and storage efficiency is a practical problem.
For example, the densification of liquid hydrogen could make it possible
to reduce the structural weight of rocket propellant tanks, thus allowing
for an increase in payload (satellite) weight. Slush hydrogen is a cryogenic
solid-liquid two-phase fluid, wherein solid hydrogen particles (particle
size of about 1 mm) are contained in liquid hydrogen, featuring greater
density, and refrigerant heat capacity than liquid hydrogen.
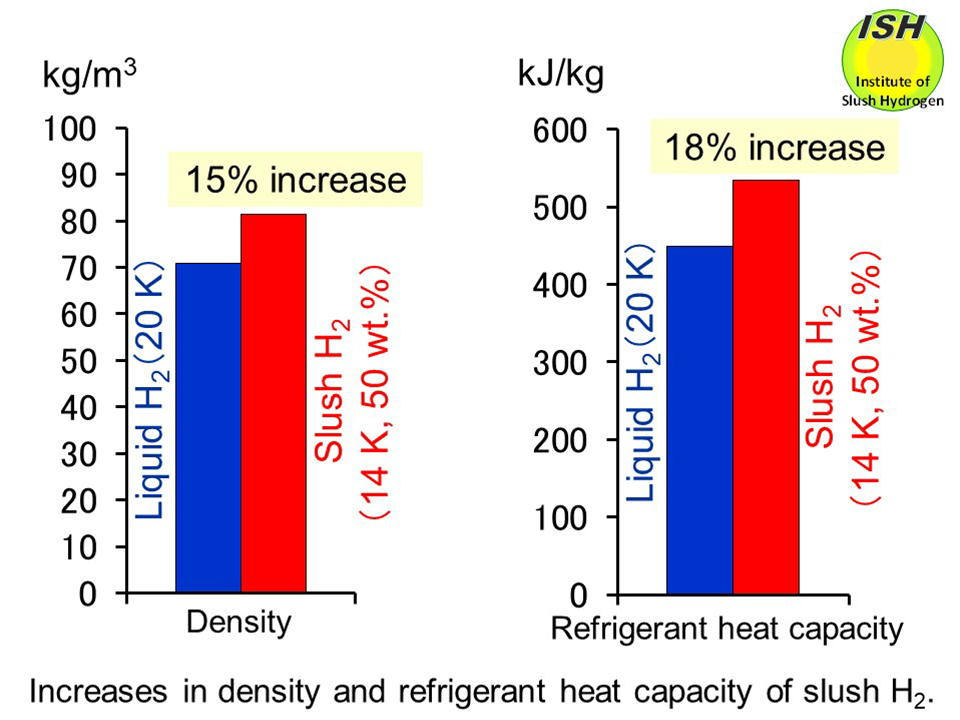
Compared to normal boiling liquid hydrogen (temperature of 20 K), slush hydrogen with a mass solid fraction of 50 wt.% (temperature of 14
K) features a 15% greater density, and an 18% increase in refrigerant heat
capacity (enthalpy) as shown in the above figure.
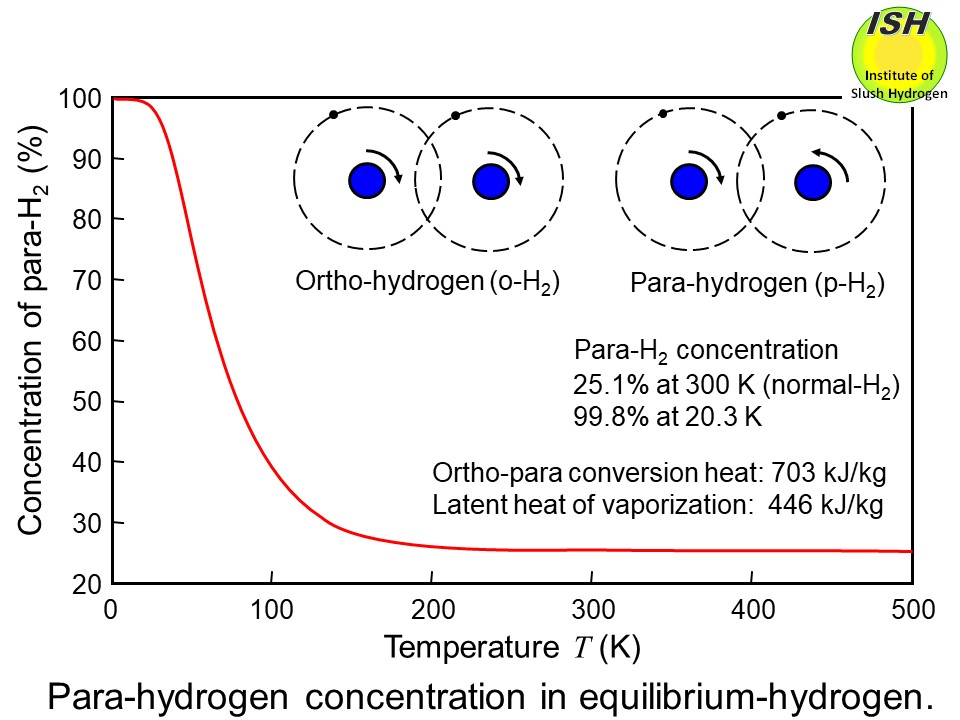
There are two different molecular forms of hydrogen: ortho-hydrogen (o-H2) and para-hydrogen (p-H2) as shown in the figure below. In the case of ortho-hydrogen, the two protons of the hydrogen molecule
spin in the same direction, while in the case of para-hydrogen, they spin
in opposite directions.
The equilibrium concentrations of ortho- and para-hydrogen differ according
to temperature. That of para-hydrogen is approximately 25% by volume at
room temperature, but nearly 100% at liquid temperature. The changeover
of ortho- to para-hydrogen generates heat of conversion of 703 kJ/kg at
the normal boiling point of hydrogen (20 K). Thus, when hydrogen gas with
a para-hydrogen concentration of 25% is liquefied and stored, ortho-para
conversion gradually occurs, generating a heat of conversion of 527 kJ/kg.
Since liquid hydrogen’s latent heat of vaporization is 446 kJ/kg, substantial
boiloff occurs during long-term storage, and storage efficiency becomes
poor.
Hydrogen liquefiers normally use a catalyst to speed up the conversion
during the liquefaction process so as to produce liquid hydrogen for storage
and transport with a para-hydrogen concentration of nearly 100%. The mixture
of ortho- and para-hydrogen at high temperatures is called normal hydrogen
(n-H2), which is a mixture of 75% ortho-hydrogen and 25% para-hydrogen, by volume.
The use of slush hydrogen would thus allow more efficient transport and
storage. Also, in the case of heat due to heat inleak or superconducting
quench (transition from a superconductiing state to a normal conducting
state), the solid fraction would be reduced due to absorption of some of
this heat by the heat of fusion associated with the solid, but liquid temperature
increase and vapor-liquid two-phase change would be inhibited. When slush
hydrogen is transported via pipeline, a solid-liquid coexisting state of
slush hydrogen can be utilized as shown in the figure below.
Another representative slush fluid is slush nitrogen, which is being considered
for use as the refrigerant for high-temperature superconducting equipment.
In this case, also at a mass solid fraction of 50 wt.% (temperature of
63 K), density is 16% higher than for normal boiling liquid nitrogen (temperature
of 77 K), while the refrigerant heat capacity (enthalpy) increases by 22%.
Slush hydrogen offers superior characteristics as a functional thermal
fluid, and various applications are anticipated for hydrogen transport
and storage [4].